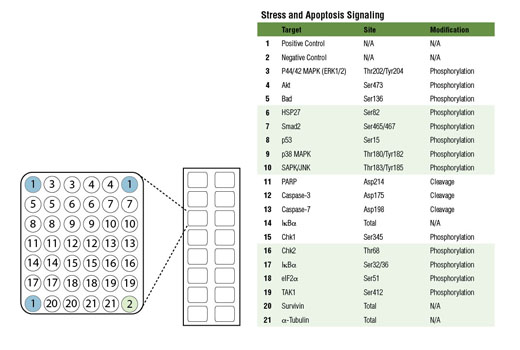
Product Information
Storage
Specificity / Sensitivity
Species Reactivity:
Human
Product Description
Background
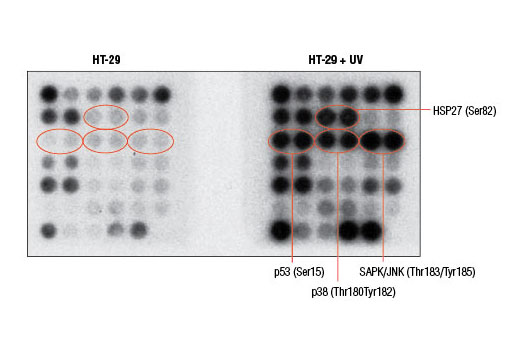
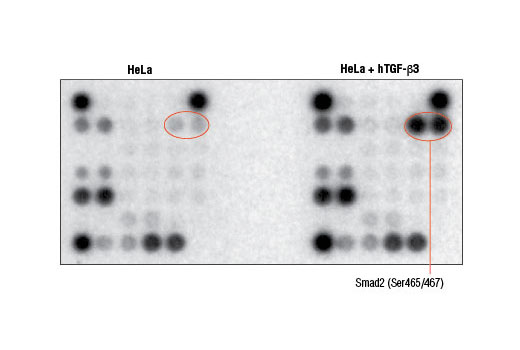
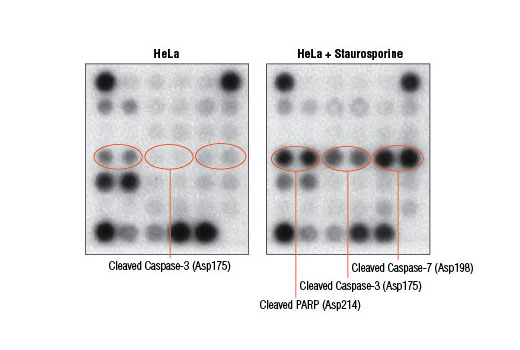
Cell death can occur due to a variety of circumstances including nutrient deprivation, inability to generate or store the energy required for metabolic functions, or deleterious environment that causes irreparable damage. Cells integrate multiple signals from a variety of sources before following either pro- or anti-apoptotic pathways. These signals can often carry conflicting information. Assessing the net effect of these processes in cell populations can be achieved by monitoring changes in a number of key signaling components. The caspase-3 and caspase-7 proteases exert a pro-apoptotic function through cleavage of multiple cellular targets. Caspase-3 and caspase-7 are activated by cleavage at Asp175 and Asp198, respectively. PARP is a DNA repair and apoptosis enzyme that is inactivated by cleavage at Asp214 by caspase-3 or caspase-7. HSP27 is a mediator of cell stress that confers resistance to adverse environmental conditions. HSP27 is activated by phosphorylation at Ser82. Chk1 and Chk2 kinases act downstream of ATM/ATR and play an important role in DNA damage checkpoint control. Activation of Chk1 and Chk2 involve phosphorylation at Ser345 and Thr68, respectively. Tumor suppressor p53 plays an important role in cellular response to DNA damage. p53 is phosphorylated at Ser15 by ATM/ATR or DNA-PK leading to its accumulation. Smad2 is a key mediator of TGF-β signaling. Stimulation by TGF-β leads to Smad2 phosphorylation at Ser465/467 and translocation of Smad2 into the nucleus. The outcome of TGF-β signaling is context dependent and can either induce apoptosis or contribute to tumor cell metastasis. Activation of NF-κB/Rel occurs through a proteasome-mediated degradation of IκBα. The inhibitor IκBα is targeted to the proteasome via phosphorylation of IκBα at Ser32 and Ser36. NF-κB activation is triggered by a diverse group of extracellular signals promoted by inflammatory cytokines, growth factors, and chemokines. TAK1 is a kinase that can be activated by TGF-β, bone morphogenetic proteins and other cytokines. Activated TAK1 phosphorylates MKK4, MKK3/6, and NIK. Phosphorylation of TAK1 at Ser412 is one of the mechanisms that regulate the levels of its activation. Cellular stress such as viral infection, endoplasmic reticulum stress, and amino acid deprivation leads to phosphorylation of eIF2α. Phosphorylation of eIF2α at Ser51 in response to cellular stress leads to a reduction of protein synthesis. The ERK1 and ERK2 MAP kinases are major signaling nodes that have many substrates and primarily transmit growth and proliferation signals. The ERK MAP kinase is activated by a dual phosphorylation of Thr202 and Tyr204. p38 MAPK and SAPK/JNK MAP kinases are activated through a similar dual phosphorylation mechanism in response to pro-inflammatory cytokines and genotoxic stress. Akt is activated by stimulation of growth-factor receptors and primarily promotes anabolic growth and survival signals via targeting its broad array of substrates. Akt phosphorylates Bad at Ser136 and inhibits its ability to induce apoptosis. Survivin is an anti-apoptotic protein that is highly expressed during fetal development and cancer cell malignancy. Survivin binds and inhibits caspase-3, controlling the cell cycle by inhibiting apoptosis and promoting cell division. α-tubulin is a building block of microtubules that are present in all eukaryotic cells. The levels of the globular α-tubulin are considered to remain relatively constant. Therefore, assessing the relative levels of α-tubulin may assist with signal normalization between the various samples.
- Boatright, K.M. and Salvesen, G.S. (2003) Curr Opin Cell Biol 15, 725-31.
- Cohen, G.M. (1997) Biochem J 326 ( Pt 1), 1-16.
- Bratton, S.B. and Cohen, G.M. (2001) Trends Pharmacol Sci 22, 306-15.
- Green, D.R. and Reed, J.C. (1998) Science 281, 1309-12.
- Basu, S. and Kolesnick, R. (1998) Oncogene 17, 3277-85.
- Jeremias, I. and Debatin, K.M. (1998) Eur Cytokine Netw 9, 687-8.
- Aravind, L. et al. (1999) Trends Biochem Sci 24, 47-53.
- Bates, S. and Vousden, K.H. (1999) Cell Mol Life Sci 55, 28-37.
- Heyninck, K. and Beyaert, R. (2001) Mol Cell Biol Res Commun 4, 259-65.
- Abe, K. et al. (2000) Ann N Y Acad Sci 926, 52-63.
- Rath, P.C. and Aggarwal, B.B. (1999) J Clin Immunol 19, 350-64.
- Holoch, P.A. and Griffith, T.S. (2009) Eur J Pharmacol 625, 63-72.
- Gjertsen, B.T. and Døskeland, S.O. (1995) Biochim Biophys Acta 1269, 187-99.
- Clemens, M.J. (2001) Prog Mol Subcell Biol 27, 57-89.
- Janes, K.A. et al. (2005) Science 310, 1646-53.
- Janes, K.A. et al. (2008) Cell 135, 343-54.
Species Reactivity
Species reactivity is determined by testing in at least one approved application (e.g., western blot).
Cross-Reactivity Key
H: human M: mouse R: rat Hm: hamster Mk: monkey Vir: virus Mi: mink C: chicken Dm: D. melanogaster X: Xenopus Z: zebrafish B: bovine Dg: dog Pg: pig Sc: S. cerevisiae Ce: C. elegans Hr: horse GP: Guinea Pig Rab: rabbit All: all species expected
Trademarks and Patents
使用に関する制限
法的な権限を与えられたCSTの担当者が署名した書面によって別途明示的に合意された場合を除き、 CST、その関連会社または代理店が提供する製品には以下の条件が適用されます。お客様が定める条件でここに定められた条件に含まれるものを超えるもの、 または、ここに定められた条件と異なるものは、法的な権限を与えられたCSTの担当者が別途書面にて受諾した場合を除き、拒絶され、 いかなる効力も効果も有しません。
研究専用 (For Research Use Only) またはこれに類似する表示がされた製品は、 いかなる目的についても FDA または外国もしくは国内のその他の規制機関により承認、認可または許可を受けていません。 お客様は製品を診断もしくは治療目的で使用してはならず、また、製品に表示された内容に違反する方法で使用してはなりません。 CST が販売または使用許諾する製品は、エンドユーザーであるお客様に対し、使途を研究および開発のみに限定して提供されるものです。 診断、予防もしくは治療目的で製品を使用することまたは製品を再販売 (単独であるか他の製品等の一部であるかを問いません) もしくはその他の商業的利用の目的で購入することについては、CST から別途許諾を得る必要があります。 お客様は以下の事項を遵守しなければなりません。(a) CST の製品 (単独であるか他の資材と一緒であるかを問いません) を販売、使用許諾、貸与、寄付もしくはその他の態様で第三者に譲渡したり使用させたりしてはなりません。また、商用の製品を製造するために CST の製品を使用してはなりません。(b) 複製、改変、リバースエンジニアリング、逆コンパイル、 分解または他の方法により製品の構造または技術を解明しようとしてはなりません。また、 CST の製品またはサービスと競合する製品またはサービスを開発する目的で CST の製品を使用してはなりません。(c) CST の製品の商標、商号、ロゴ、特許または著作権に関する通知または表示を除去したり改変したりしてはなりません。(d) CST の製品をCST 製品販売条件(CST’s Product Terms of Sale) および該当する書面のみに従って使用しなければなりません。(e) CST の製品に関連してお客様が使用する第三者の製品またはサービスに関する使用許諾条件、 サービス提供条件またはこれに類する合意事項を遵守しなければなりません。