| Product Includes | Product # | Quantity | Color | Storage Temp |
|---|---|---|---|---|
| ALK Rabbit mAb Coated Microwells | 64463 | 96 tests |
|
+4C |
| ALK Mouse Detection mAb | 13993 | 1 ea |
|
+4C |
| Anti-mouse IgG, HRP-linked Antibody (ELISA Formulated) | 13304 | 1 ea |
|
+4C |
| Detection Antibody Diluent | 13339 | 11 ml |
|
+4C |
| HRP Diluent | 13515 | 11 ml |
|
+4C |
| TMB Substrate | 7004 | 11 ml |
|
+4C |
| STOP Solution | 7002 | 11 ml |
|
+4C |
| Sealing Tape | 54503 | 2 ea |
|
+4C |
| ELISA Wash Buffer (20X) | 9801 | 25 ml |
|
+4C |
| ELISA Sample Diluent | 11083 | 25 ml |
|
+4C |
| Cell Lysis Buffer (10X) | 9803 | 15 ml |
|
-20C |
*The microwell plate is supplied as 12 8-well modules - Each module is designed to break apart for 8 tests.
Description
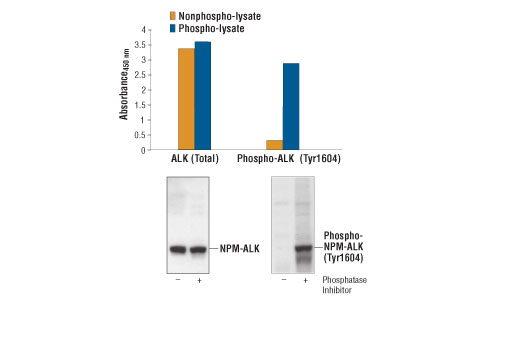
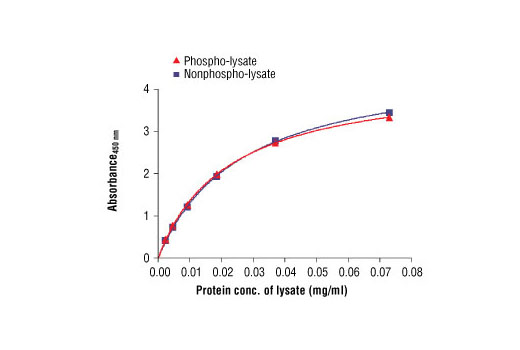
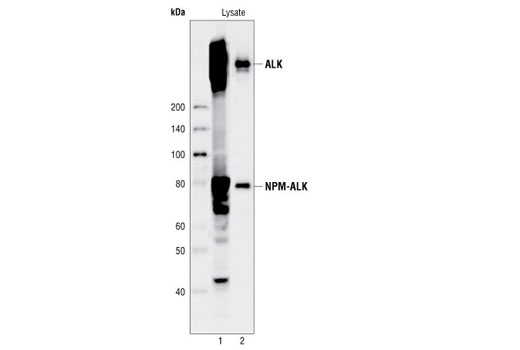
CST's PathScan® Total ALK Sandwich ELISA Kit is a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) that detects endogenous levels of total ALK and NPM-ALK fusion protein. An ALK rabbit capture antibody has been coated onto the microwells. After incubation with cell lysates, ALK and NPM-ALK proteins (phospho and nonphospho) are captured by the coated antibody. Following extensive washing, an ALK mouse detection antibody is added to detect the captured ALK and NPM-ALK proteins. Anti-mouse IgG, HRP-linked Antibody is then used to recognize the bound detection antibody. HRP substrate, TMB, is added to develop color. The magnitude of absorbance for this developed color is proportional to the quantity of total ALK and NPM-ALK proteins.
*Antibodies in kit are custom formulations specific to kit.
Specificity/Sensitivity
* Phosphatase inhibitors include sodium pyrophosphate, β-glycerophosphate and Na3VO4.
Background
Anaplastic lymphoma kinase (ALK) is a tyrosine kinase receptor for pleiotrophin (PTN), a growth factor involved in embryonic brain development (1-3). In ALK-expressing cells, PTN induces phosphorylation of both ALK and the downstream effectors IRS-1, Shc, PLCγ, and PI3 kinase (1). ALK was originally discovered as a nucleophosmin (NPM)-ALK fusion protein produced by a translocation (4). Investigators have found that the NPM-ALK fusion protein is a constitutively active, oncogenic tyrosine kinase associated with anaplastic lymphoma (4). Research literature suggests that activation of PLCγ by NPM-ALK may be a crucial step for its mitogenic activity and involved in the pathogenesis of anaplastic lymphomas (5).A distinct ALK oncogenic fusion protein involving ALK and echinoderm microtubule-associated protein like 4 (EML4) has been described in the research literature from a non-small cell lung cancer (NSCLC) cell line, with corresponding fusion transcripts present in some cases of lung adenocarcinoma. The short, amino-terminal region of the microtubule-associated protein EML4 is fused to the kinase domain of ALK (6-8).
- Stoica, G.E. et al. (2001) J Biol Chem 276, 16772-9.
- Iwahara, T. et al. (1997) Oncogene 14, 439-49.
- Morris, S.W. et al. (1997) Oncogene 14, 2175-88.
- Morris, S.W. et al. (1994) Science 263, 1281-4.
- Bai, R.Y. et al. (1998) Mol Cell Biol 18, 6951-61.
- Rikova, K. et al. (2007) Cell 131, 1190-203.
- Takeuchi, K. et al. (2008) Clin Cancer Res 14, 6618-24.
- Soda, M. et al. (2007) Nature 448, 561-6.
Background References
Cross-Reactivity Key
H: human M: mouse R: rat Hm: hamster Mk: monkey Vir: virus Mi: mink C: chicken Dm: D. melanogaster X: Xenopus Z: zebrafish B: bovine Dg: dog Pg: pig Sc: S. cerevisiae Ce: C. elegans Hr: horse GP: Guinea Pig Rab: rabbit All: all species expected
Trademarks and Patents
使用に関する制限
法的な権限を与えられたCSTの担当者が署名した書面によって別途明示的に合意された場合を除き、 CST、その関連会社または代理店が提供する製品には以下の条件が適用されます。お客様が定める条件でここに定められた条件に含まれるものを超えるもの、 または、ここに定められた条件と異なるものは、法的な権限を与えられたCSTの担当者が別途書面にて受諾した場合を除き、拒絶され、 いかなる効力も効果も有しません。
研究専用 (For Research Use Only) またはこれに類似する表示がされた製品は、 いかなる目的についても FDA または外国もしくは国内のその他の規制機関により承認、認可または許可を受けていません。 お客様は製品を診断もしくは治療目的で使用してはならず、また、製品に表示された内容に違反する方法で使用してはなりません。 CST が販売または使用許諾する製品は、エンドユーザーであるお客様に対し、使途を研究および開発のみに限定して提供されるものです。 診断、予防もしくは治療目的で製品を使用することまたは製品を再販売 (単独であるか他の製品等の一部であるかを問いません) もしくはその他の商業的利用の目的で購入することについては、CST から別途許諾を得る必要があります。 お客様は以下の事項を遵守しなければなりません。(a) CST の製品 (単独であるか他の資材と一緒であるかを問いません) を販売、使用許諾、貸与、寄付もしくはその他の態様で第三者に譲渡したり使用させたりしてはなりません。また、商用の製品を製造するために CST の製品を使用してはなりません。(b) 複製、改変、リバースエンジニアリング、逆コンパイル、 分解または他の方法により製品の構造または技術を解明しようとしてはなりません。また、 CST の製品またはサービスと競合する製品またはサービスを開発する目的で CST の製品を使用してはなりません。(c) CST の製品の商標、商号、ロゴ、特許または著作権に関する通知または表示を除去したり改変したりしてはなりません。(d) CST の製品をCST 製品販売条件(CST’s Product Terms of Sale) および該当する書面のみに従って使用しなければなりません。(e) CST の製品に関連してお客様が使用する第三者の製品またはサービスに関する使用許諾条件、 サービス提供条件またはこれに類する合意事項を遵守しなければなりません。