| Product Includes | Product # | Quantity | Mol. Wt | Isotype/Source |
|---|---|---|---|---|
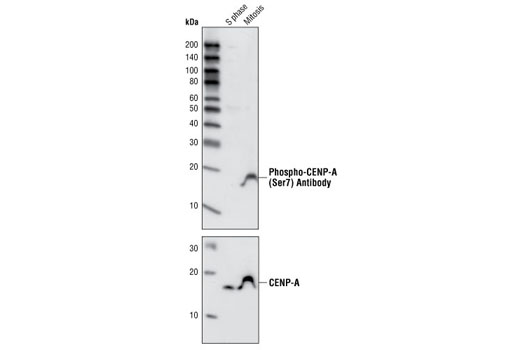
| Phospho-CENP-A (Ser7) Antibody | 2187 | 20 µl | 17 kDa | Rabbit |
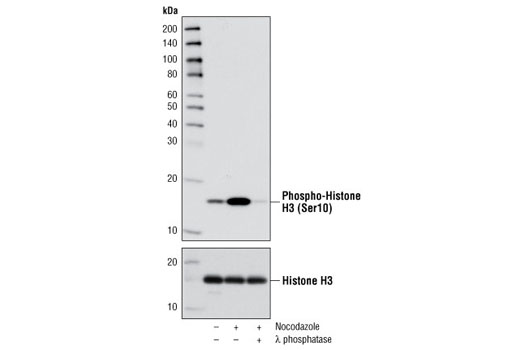
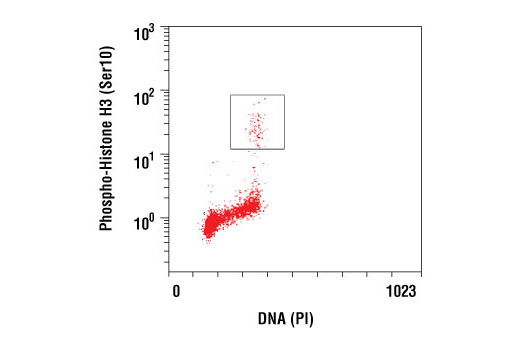
| Phospho-Histone H3 (Ser10) (D2C8) XP® Rabbit mAb | 3377 | 20 µl | 17 kDa | Rabbit IgG |
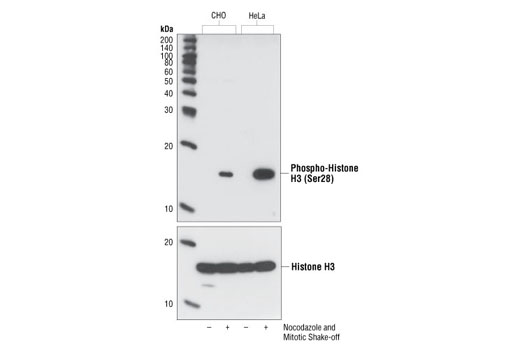
| Phospho-Histone H3 (Ser28) Antibody | 9713 | 20 µl | 17 kDa | Rabbit |
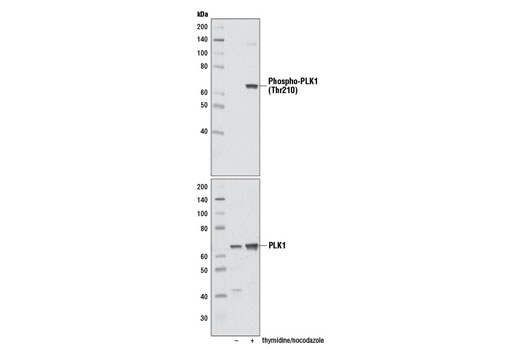
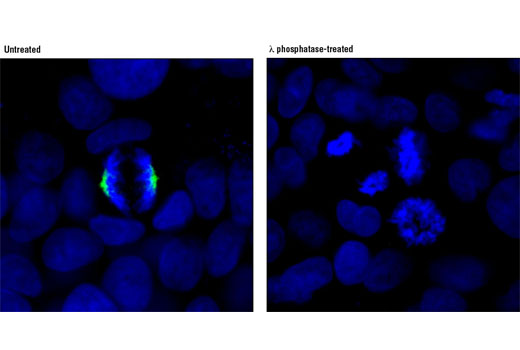
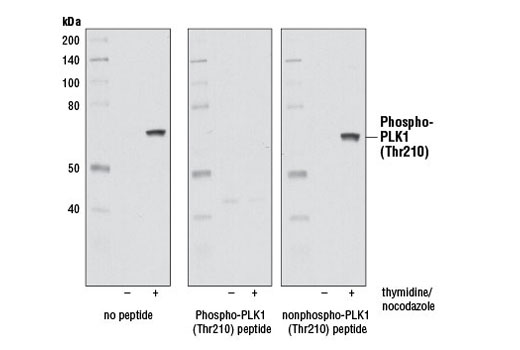
| Phospho-PLK1 (Thr210) (D5H7) Rabbit mAb | 9062 | 20 µl | 62 kDa | Rabbit IgG |
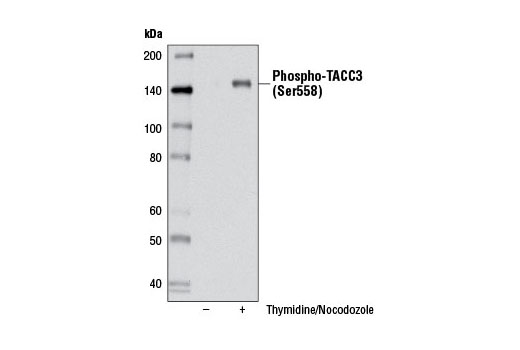
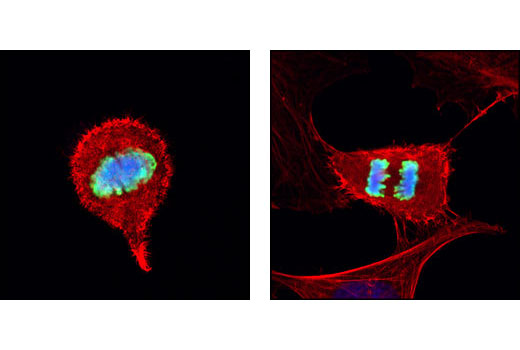
| Phospho-TACC3 (Ser558) (D8H10) XP® Rabbit mAb | 8842 | 20 µl | 140 kDa | Rabbit IgG |
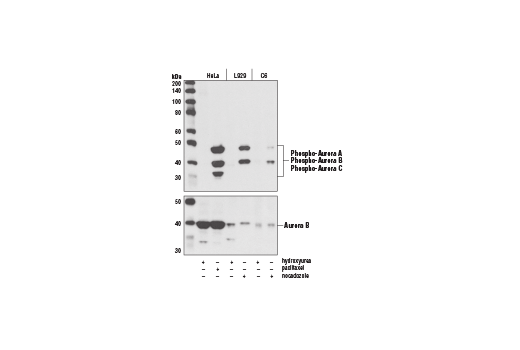
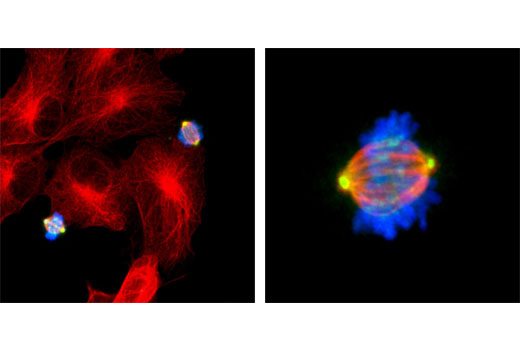
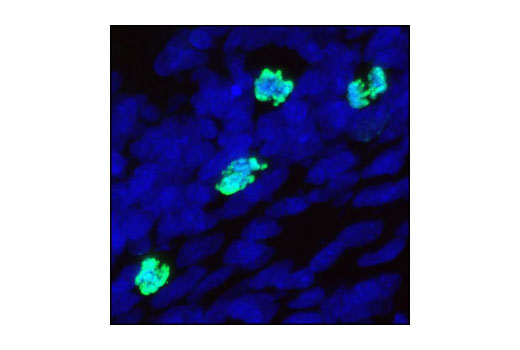
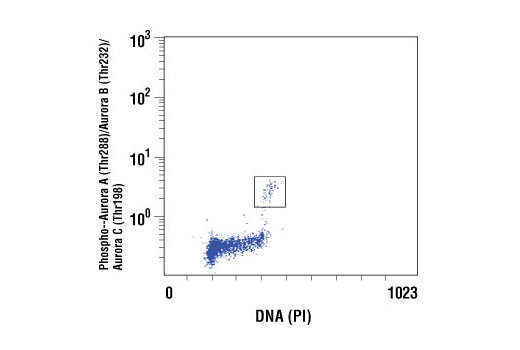
| Phospho-Aurora A (Thr288)/Aurora B (Thr232)/Aurora C (Thr198) (D13A11) XP® Rabbit mAb | 2914 | 20 µl | 35, 40, 48 kDa | Rabbit IgG |
| Anti-rabbit IgG, HRP-linked Antibody | 7074 | 100 µl | Goat |
Please visit cellsignal.com for individual component applications, species cross-reactivity, dilutions, protocols, and additional product information.
Description
The Aurora A/B Substrate Antibody Sampler Kit provides an economical means to investigate the G2/M phase of the cell cycle. The kit includes enough antibodies to perform two western blot experiments with each primary antibody.
Storage
Background
Aurora kinases belong to a highly conserved family of mitotic serine/threonine kinases with three members identified among mammals: Aurora A, B, and C (1,2). Studies on the temporal expression pattern and subcellular localization of Aurora kinases in mitotic cells suggest an association with mitotic structure. Aurora kinase functional influences span from G2 phase to cytokinesis and may be involved in key cell cycle events such as centrosome duplication, chromosome bi-orientation and segregation, cleavage furrow positioning, and ingression (3). Aurora A is detected at the centrosomes, along mitotic spindle microtubules, and in the cytoplasm of mitotically proliferating cells. Aurora A protein levels are low during G1 and S phases and peak during the G2/M phase of the cell cycle. Phosphorylation of Aurora A at Thr288 in its catalytic domain increases kinase activity. Aurora A is involved in centrosome separation, maturation, and spindle assembly and stability. Expression of Aurora B protein also peaks during the G2/M phase of the cell cycle; Aurora B kinase activity peaks at the transition from metaphase to the end of mitosis. Aurora B associates with chromosomes during prophase prior to relocalizing to the spindle at anaphase. Aurora B regulates chromosome segregation through the control of microtubule-kinetochore attachment and cytokinesis. Expression of both Aurora A and Aurora B during the G2/M phase transition is tightly coordinated with histone H3 phosphorylation (4,5); research investigators have observed overexpression of these kinases in a variety of human cancers (2,4). Aurora C localizes to the centrosome from anaphase to cytokinesis and both mRNA and protein levels peak during G2/M phase. Although typical Aurora C expression is limited to the testis, research studies report overexpression of Aurora C is detected in various cancer cell lines (6).
Transforming acid coiled-coil (TACC) proteins are a family of proteins characterized by a common coiled-coil motif of approximately 200 amino acids at the carboxy-terminal end (7). When phosphorylated at Ser558 by Aurora A, mammalian TACC3 is localized to mitotic spindles and increases microtubule stability (8,9).
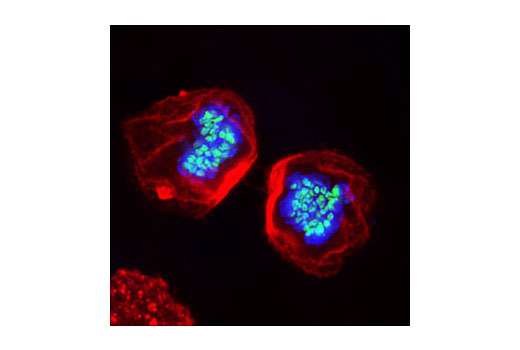
Aurora A-dependent phosphorylation of CENP-A on Ser7 during prophase is required for proper targeting of Aurora B to the inner centromere in prometaphase, proper kinetochore/microtubule attachment, and proper alignment of chromosomes during mitosis (10). Aurora B also targets Ser7 on CENP-A, which in turn regulates Aurora B activity during cytokinesis (11). Aurora B phosphorylates both Ser10 and Ser28 on histone H3 in concordance with mitotic chromosome condensation (12).
Aurora A phosphorylation of Thr210 on PLK promotes mitotic entry following checkpoint-dependent cell cycle arrest (13). Autophosphorylation of conserved threonine residues Thr288 (Aurora A), Thr232 (Aurora B), and Thr195 (Aurora C) is required for Aurora kinase activity (14).
- Warner, S.L. et al. (2003) Mol Cancer Ther 2, 589-95.
- Katayama, H. et al. (2003) Cancer Metastasis Rev 22, 451-64.
- Andrews, P.D. et al. (2003) Curr Opin Cell Biol 15, 672-83.
- Pascreau, G. et al. (2003) Prog Cell Cycle Res 5, 369-74.
- Crosio, C. et al. (2002) Mol Cell Biol 22, 874-85.
- Kimura, M. et al. (1999) J Biol Chem 274, 7334-40.
- Gergely, F. et al. (2000) Proc Natl Acad Sci U S A 97, 14352-7.
- Kinoshita, K. et al. (2005) J Cell Biol 170, 1047-55.
- Schneider, L. et al. (2007) J Biol Chem 282, 29273-83.
- Kunitoku, N. et al. (2003) Dev Cell 5, 853-64.
- Zeitlin, S.G. et al. (2001) J Cell Biol 155, 1147-57.
- Goto, H. et al. (2002) Genes Cells 7, 11-7.
- Macůrek, L. et al. (2008) Nature 455, 119-23.
- Willems, E. et al. (2018) Cell Div 13, 7.
Background References
Trademarks and Patents
使用に関する制限
法的な権限を与えられたCSTの担当者が署名した書面によって別途明示的に合意された場合を除き、 CST、その関連会社または代理店が提供する製品には以下の条件が適用されます。お客様が定める条件でここに定められた条件に含まれるものを超えるもの、 または、ここに定められた条件と異なるものは、法的な権限を与えられたCSTの担当者が別途書面にて受諾した場合を除き、拒絶され、 いかなる効力も効果も有しません。
研究専用 (For Research Use Only) またはこれに類似する表示がされた製品は、 いかなる目的についても FDA または外国もしくは国内のその他の規制機関により承認、認可または許可を受けていません。 お客様は製品を診断もしくは治療目的で使用してはならず、また、製品に表示された内容に違反する方法で使用してはなりません。 CST が販売または使用許諾する製品は、エンドユーザーであるお客様に対し、使途を研究および開発のみに限定して提供されるものです。 診断、予防もしくは治療目的で製品を使用することまたは製品を再販売 (単独であるか他の製品等の一部であるかを問いません) もしくはその他の商業的利用の目的で購入することについては、CST から別途許諾を得る必要があります。 お客様は以下の事項を遵守しなければなりません。(a) CST の製品 (単独であるか他の資材と一緒であるかを問いません) を販売、使用許諾、貸与、寄付もしくはその他の態様で第三者に譲渡したり使用させたりしてはなりません。また、商用の製品を製造するために CST の製品を使用してはなりません。(b) 複製、改変、リバースエンジニアリング、逆コンパイル、 分解または他の方法により製品の構造または技術を解明しようとしてはなりません。また、 CST の製品またはサービスと競合する製品またはサービスを開発する目的で CST の製品を使用してはなりません。(c) CST の製品の商標、商号、ロゴ、特許または著作権に関する通知または表示を除去したり改変したりしてはなりません。(d) CST の製品をCST 製品販売条件(CST’s Product Terms of Sale) および該当する書面のみに従って使用しなければなりません。(e) CST の製品に関連してお客様が使用する第三者の製品またはサービスに関する使用許諾条件、 サービス提供条件またはこれに類する合意事項を遵守しなければなりません。