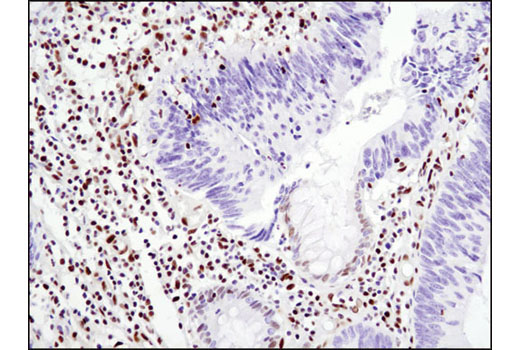
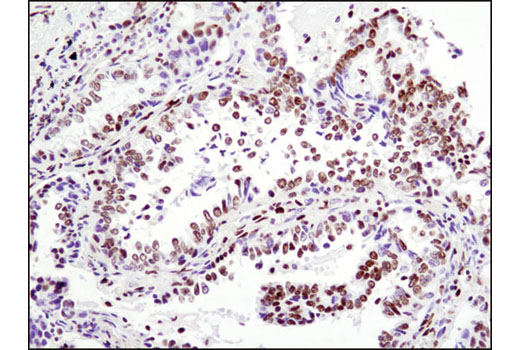
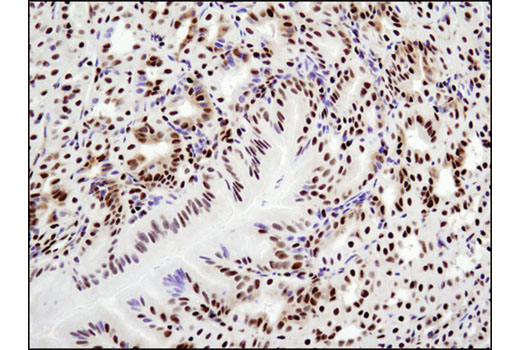
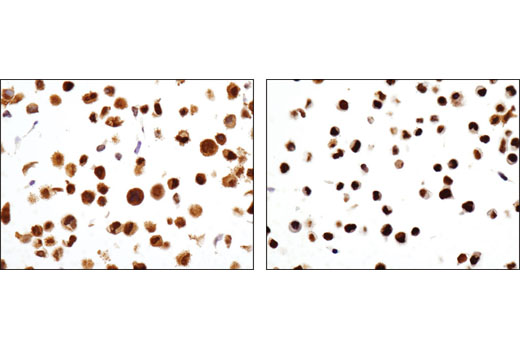
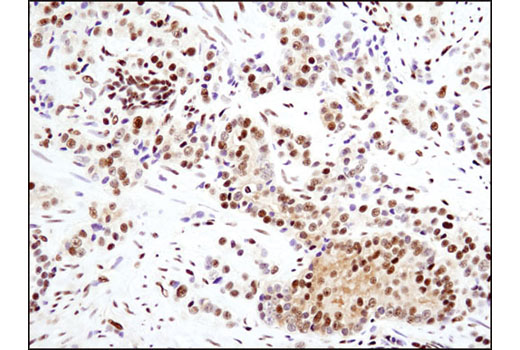
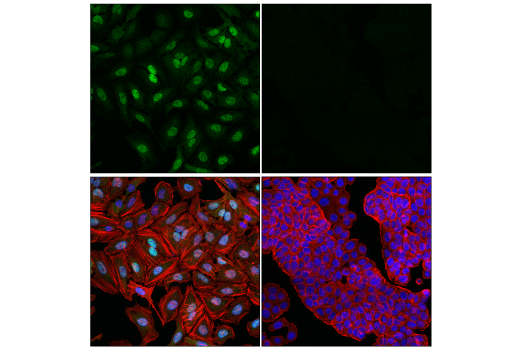
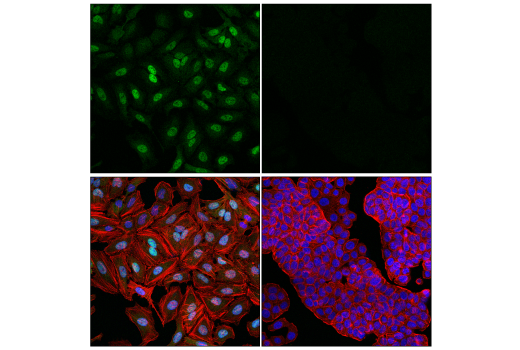
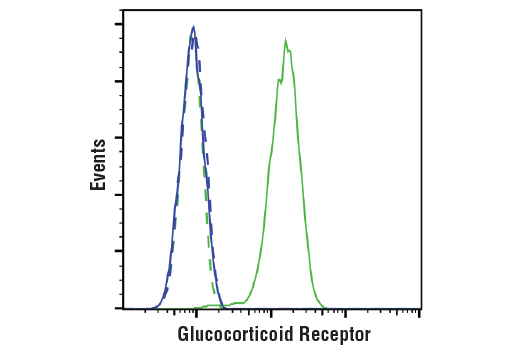
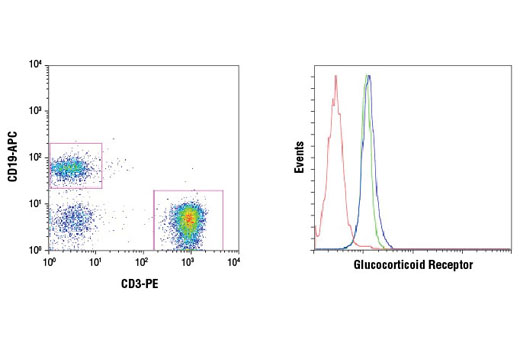
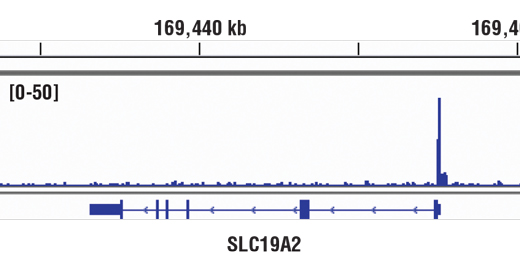
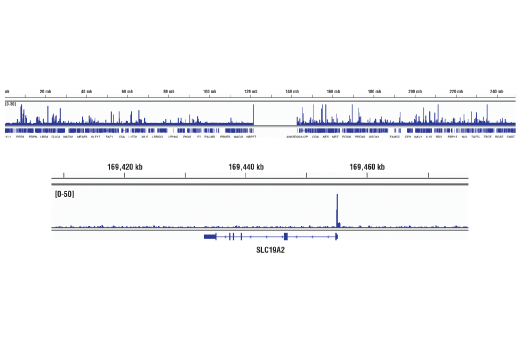
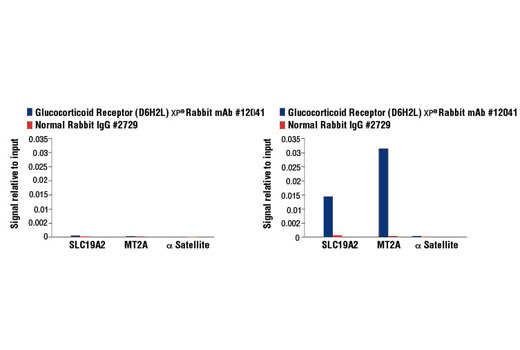
WB, IP, IHC-P, IF-IC, FC-FP, ChIP, ChIP-seq, C&R
H M R Mk
Endogenous
94, 91
Rabbit IgG
#P04150
2908
Product Information
Product Usage Information
The CUT&RUN dilution was determined using CUT&RUN Assay Kit #86652.
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Immunoprecipitation | 1:100 |
| Immunohistochemistry (Paraffin) | 1:200 - 1:800 |
| Immunofluorescence (Immunocytochemistry) | 1:800 - 1:1600 |
| Flow Cytometry (Fixed/Permeabilized) | 1:50 - 1:200 |
| Chromatin IP | 1:50 |
| Chromatin IP-seq | 1:50 |
| CUT&RUN | 1:50 |
Storage
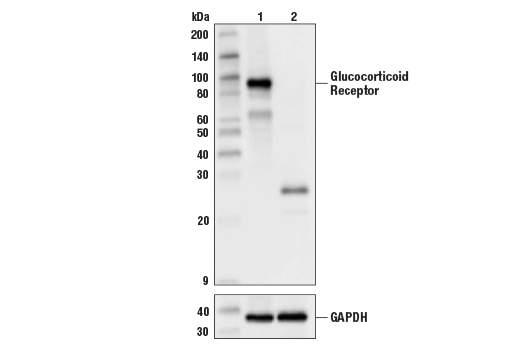
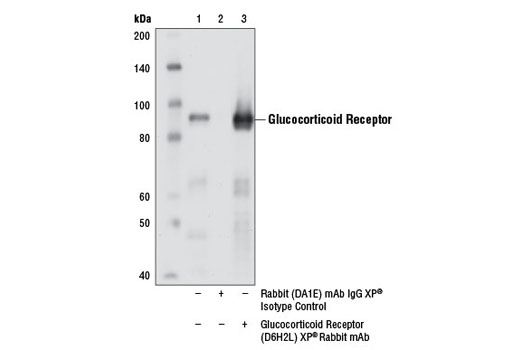
Specificity / Sensitivity
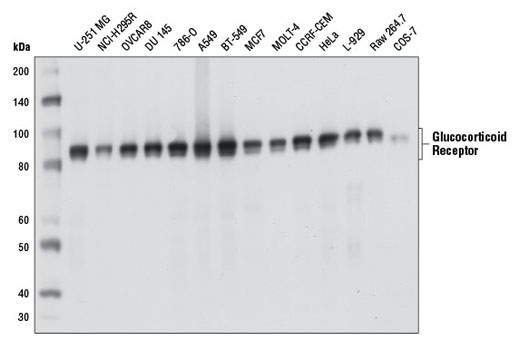
Species Reactivity:
Human, Mouse, Rat, Monkey
Source / Purification
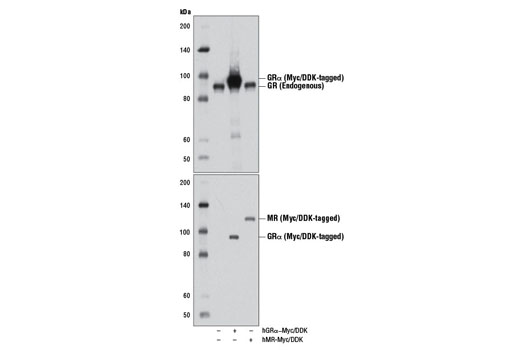
Monoclonal antibody is produced by immunizing animals with a recombinant protein specific to the amino terminus of human GR protein.
Background
Glucocorticoid hormones control cellular proliferation, inflammation, and metabolism through their association with the glucocorticoid receptor (GR)/NR3C1, a member of the nuclear hormone receptor superfamily of transcription factors (1). GR is composed of several conserved structural elements, including a carboxy-terminal ligand-binding domain (which also contains residues critical for receptor dimerization and hormone-dependent gene transactivation), a neighboring hinge region containing nuclear localization signals, a central zinc-finger-containing DNA-binding domain, and an amino-terminal variable region that participates in ligand-independent gene transcription. In the absence of hormone, a significant population of GR is localized to the cytoplasm in an inactive form via its association with regulatory chaperone proteins, such as HSP90, HSP70, and FKBP52. On hormone binding, GR is released from the chaperone complex and translocates to the nucleus as a dimer to associate with specific DNA sequences termed glucocorticoid response elements (GREs), thereby enhancing or repressing transcription of specific target genes (2). It was demonstrated that GR-mediated transcriptional activation is modulated by phosphorylation (3-5). Although GR can be basally phosphorylated in the absence of hormone, it becomes hyperphosphorylated upon binding receptor agonists. It has been suggested that hormone-dependent phosphorylation of GR may determine target promoter specificity, cofactor interaction, strength and duration of receptor signaling, receptor stability, and receptor subcellular localization (3).
Species Reactivity
Species reactivity is determined by testing in at least one approved application (e.g., western blot).
Western Blot Buffer
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 5% w/v BSA, 1X TBS, 0.1% Tween® 20 at 4°C with gentle shaking, overnight.
Applications Key
WB: Western Blotting IP: Immunoprecipitation IHC-P: Immunohistochemistry (Paraffin) IF-IC: Immunofluorescence (Immunocytochemistry) FC-FP: Flow Cytometry (Fixed/Permeabilized) ChIP: Chromatin IP ChIP-seq: Chromatin IP-seq C&R: CUT&RUN
Cross-Reactivity Key
H: human M: mouse R: rat Hm: hamster Mk: monkey Vir: virus Mi: mink C: chicken Dm: D. melanogaster X: Xenopus Z: zebrafish B: bovine Dg: dog Pg: pig Sc: S. cerevisiae Ce: C. elegans Hr: horse GP: Guinea Pig Rab: rabbit All: all species expected
Trademarks and Patents
使用に関する制限
法的な権限を与えられたCSTの担当者が署名した書面によって別途明示的に合意された場合を除き、 CST、その関連会社または代理店が提供する製品には以下の条件が適用されます。お客様が定める条件でここに定められた条件に含まれるものを超えるもの、 または、ここに定められた条件と異なるものは、法的な権限を与えられたCSTの担当者が別途書面にて受諾した場合を除き、拒絶され、 いかなる効力も効果も有しません。
研究専用 (For Research Use Only) またはこれに類似する表示がされた製品は、 いかなる目的についても FDA または外国もしくは国内のその他の規制機関により承認、認可または許可を受けていません。 お客様は製品を診断もしくは治療目的で使用してはならず、また、製品に表示された内容に違反する方法で使用してはなりません。 CST が販売または使用許諾する製品は、エンドユーザーであるお客様に対し、使途を研究および開発のみに限定して提供されるものです。 診断、予防もしくは治療目的で製品を使用することまたは製品を再販売 (単独であるか他の製品等の一部であるかを問いません) もしくはその他の商業的利用の目的で購入することについては、CST から別途許諾を得る必要があります。 お客様は以下の事項を遵守しなければなりません。(a) CST の製品 (単独であるか他の資材と一緒であるかを問いません) を販売、使用許諾、貸与、寄付もしくはその他の態様で第三者に譲渡したり使用させたりしてはなりません。また、商用の製品を製造するために CST の製品を使用してはなりません。(b) 複製、改変、リバースエンジニアリング、逆コンパイル、 分解または他の方法により製品の構造または技術を解明しようとしてはなりません。また、 CST の製品またはサービスと競合する製品またはサービスを開発する目的で CST の製品を使用してはなりません。(c) CST の製品の商標、商号、ロゴ、特許または著作権に関する通知または表示を除去したり改変したりしてはなりません。(d) CST の製品をCST 製品販売条件(CST’s Product Terms of Sale) および該当する書面のみに従って使用しなければなりません。(e) CST の製品に関連してお客様が使用する第三者の製品またはサービスに関する使用許諾条件、 サービス提供条件またはこれに類する合意事項を遵守しなければなりません。